Champix (Varenicline)











Dosages
Champix 1 mg
| Quantity | Price per item | Total price | |
|---|---|---|---|
| 28 | £3.80 | £106.54 | |
| 56 | £3.30 | £184.97 | |
| 84 | £3.07 | £258.21 |
Payment & Delivery
Your order is carefully packed and dispatched within 24 hours. Here's the typical package.
Roughly the size of a standard letter (24x11x0.7 cm), with no indication of its contents.



| Delivery Method | Estimated delivery |
|---|---|
| Express Free on orders over £221.96 | Estimated delivery to the UK: 4-7 days |
| Standard Free on orders over £147.97 | Estimated delivery to the UK: 14-21 days |










Discount Coupons
- New Year's Day - 1 January 2025 9% NEWYEAR9
- Valentine's Day - 14 February 2025 6% VALENTINE6
- St David's Day - 1 March 2025 4% DAVID4
- St Patrick's Day - 17 March 2025 5% PATRICK5
- St George's Day - 23 April 2025 4% GEORGE4
- May Day - 1 May 2025 5% MAYDAY5
- Halloween - 31 October 2025 6% HALLOWEEN6
- Guy Fawkes Night - 5 November 2025 5% GUY5
- Christmas Eve - 24 December 2025 9% XMASEVE9
- Christmas Day - 25 December 2025 10% XMAS10
- Boxing Day - 26 December 2025 10% BOXING10
- New Year's Eve - 31 December 2025 8% NYE8
FAQ
Description
Champix (generic name: Varenicline) is a prescription medication used to help people quit smoking. It is primarily used as a smoking cessation aid and is intended to be combined with behavioral support to increase the chances of successfully quitting smoking.
It comes in the form of tablets and is available in two strengths: 0,5mg and 1mg. The treatment usually starts with 0,5mg tablets; after a certain period, the dosage is increased to 1mg.

Following the prescribed dosing schedule and consulting a healthcare professional before starting Champix is essential. The medication works by targeting the nicotine receptors in the brain, which helps reduce withdrawal symptoms and cravings associated with quitting smoking. However, this drug may have side effects like all medications, and not everyone may be suitable for its use. Therefore, discussing any existing medical conditions or medications with a healthcare provider before starting Champix is crucial.
Content
- Each 0,5 mg film-coated tablet contains 0,5 mg of varenicline (as tartrate).
- Each 1 mg film-coated tablet contains 1 mg of varenicline (as tartrate).
- Excipients
How the Pills Look Like
The medicine is available in two strengths with distinct characteristics:
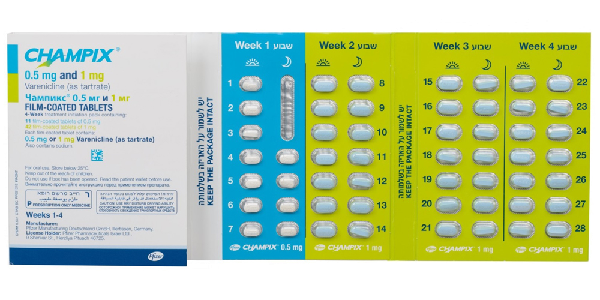
- 0,5mg tablets: These film-coated tablets are white, capsular-shaped, and biconvex, measuring approximately 4mm x 8mm. One side is imprinted with "Pfizer," while the other side bears the marking "CHX 0.5."
- 1mg tablets: These film-coated tablets come in light blue, capsular-shaped, and biconvex, with dimensions of about 5mm x 10mm. Like the 0.5mg version, one side displays the word "Pfizer," while the other is embossed with "CHX 1.0."
Mechanism of Action
Champix (Varenicline) tablets work as a smoking cessation aid by targeting nicotine receptors in the brain. Nicotine is the addictive substance found in cigarettes and other tobacco products, and it binds to specific receptors in the brain, triggering the release of dopamine, which creates feelings of pleasure and reward.
Varenicline, the active ingredient in this drug, works in two ways:
- Partial Agonist: Varenicline acts as a partial agonist at nicotine receptors. This means it stimulates these receptors to a lesser extent than nicotine. By partially activating the receptors, varenicline helps reduce withdrawal symptoms and cravings associated with quitting smoking.
- Blocking Effects: Varenicline also has a blocking effect on nicotine receptors. It competes with nicotine to bind to these receptors and, once bound, prevents nicotine from fully activating them. This diminishes the rewarding effects of smoking, making it less satisfying if a person relapses and smokes while taking this medicine.
By combining these mechanisms, Champix helps reduce the desire to smoke and minimize the withdrawal symptoms that often make quitting smoking challenging. It also helps break the association between smoking and the pleasurable feelings typically associated with nicotine intake.
Dosage
This drug is intended for oral administration, and the tablets should be ingested whole with water. It can be taken with or without food.
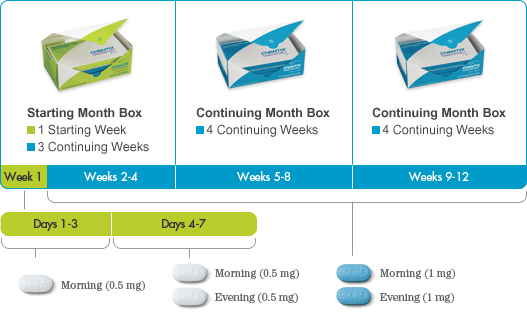
The recommended dose is 1 mg varenicline twice daily following a 1-week titration as follows:
| Days 1 - 3: | 0.5 mg once daily |
| Days 4 - 7: | 0.5 mg twice daily |
| Day 8 - End of treatment: | 1 mg twice daily |
To begin a smoking cessation treatment with Champix (Varenicline), the patient should select a specific quit date. Typically, dosing should commence 1-2 weeks before this chosen quit date. The standard treatment duration is 12 weeks.
For individuals who successfully quit smoking by the end of the initial 12-week treatment period, there is an option for an additional 12 weeks of maintenance therapy with Champix at a dosage of 1 mg twice daily to help maintain abstinence.
In cases where patients find it challenging to quit abruptly, a gradual approach may be considered. They can start reducing smoking during the first 12 weeks of treatment and aim to quit entirely by the end of this period. After that, they can continue taking the drug for another 12 weeks, totaling 24 weeks of treatment.
For patients who remain motivated to quit smoking but failed in their previous Champix therapy or experienced a relapse after treatment, another quit attempt with this medicine may be beneficial.
If a patient experiences adverse reactions to this drug, they have the option to temporarily or permanently lower the dose to 0,5 mg twice daily.
After the cessation treatment ends, there is an increased risk of relapse to smoking. In patients with a higher risk of relapse, dose tapering may be considered as part of the smoking cessation therapy.
Elderly Patients
Dosage adjustment is not required for elderly patients. However, since elderly patients may have reduced renal function, prescribers should consider the patient's renal status.
Children
This medicine is not recommended for pediatric patients as its efficacy in this age group has not been demonstrated.
Renal Impairment
Patients with mild to moderate renal impairment (estimated creatinine clearance > 50 ml/min and ≤ 80 ml/min or ≥ 30 ml/min and ≤ 50 ml/min) do not require dosage adjustment.
For patients with moderate renal impairment who experience intolerable adverse reactions, the dose may be reduced to 1 mg once daily.
For patients with severe renal impairment (estimated creatinine clearance < 30 ml/min), the recommended dose is 1 mg once daily. Initially, dosing should begin at 0.5 mg once daily for the first three days and then increased to 1 mg once daily. However, there is insufficient clinical experience with Champix in patients with end-stage renal disease, and therefore, its use is not recommended in this patient population.
Hepatic Impairment
Dosage adjustment is not necessary for patients with hepatic impairment.
Side Effects
Like all medications, Champix (Varenicline) 0,5mg and 1mg tablets may cause side effects in some individuals. Common side effects can include:
- nausea;
- headache;
- insomnia (difficulty sleeping);
- abnormal dreams;
- changes in taste perception;
- dry mouth;
- constipation;
- fatigue or tiredness.
Not everyone will experience these side effects, and some individuals may react differently to the medication. Additionally, some people may experience more severe side effects, although they are less common. These potentially serious side effects may include:
- mood changes, such as depression or anxiety;
- suicidal thoughts or behaviors;
- aggressive behavior;
- hallucinations or psychosis;
- allergic reactions with symptoms like rash, itching, swelling, severe dizziness, or difficulty breathing.
If you or someone you know experiences any concerning or severe side effects while taking this drug, it is crucial to seek immediate medical attention.
It is essential to follow the prescribed dosage and inform your healthcare provider about any pre-existing medical conditions or medications you are taking to reduce the risk of adverse reactions. Your healthcare provider can monitor your progress and adjust the treatment plan if necessary to manage side effects effectively.
Special Warnings and Precautions for Use
Here is the warning information for those starting treatment with this medication.
Effect of Smoking Cessation
When a person quits smoking, whether with or without Champix treatment, physiological changes can affect how certain medications are processed in the body (pharmacokinetics) and how they exert their effects (pharmacodynamics). This may require dosage adjustments for specific medicinal products. Examples of such medications include theophylline, warfarin, and insulin.
Smoking is known to induce CYP1A2, an enzyme involved in drug metabolism. Therefore, smoking cessation may lead to increased plasma levels of CYP1A2 substrates, potentially influencing their efficacy or safety. Healthcare providers must be mindful of these potential changes and consider the need for dosage modifications when patients undergo smoking cessation.
Neuropsychiatric Symptoms
Champix use for smoking cessation has been associated with reports of changes in behavior, mood swings, anxiety, depression, and even suicidal thoughts and actions in post-marketing experiences. However, a comprehensive study comparing patients with and without a history of psychiatric disorders using varenicline, bupropion, nicotine replacement therapy patch (NRT), or placebo did not show an increased risk of serious neuropsychiatric events with varenicline compared to placebo.
A depressed mood, including suicidal thoughts, can be a symptom of nicotine withdrawal. Clinicians should be vigilant about the emergence of serious neuropsychiatric symptoms in patients attempting to quit smoking, with or without medication. If such symptoms occur during varenicline treatment, patients should stop using varenicline immediately and seek medical evaluation.
In summary, while there have been reports of neuropsychiatric side effects, a rigorous study did not find an increased risk with varenicline compared to placebo. Nevertheless, clinicians should be cautious and monitor patients closely during smoking cessation treatment.
History of Psychiatric Disorders
Individuals with a history of psychiatric disorders should exercise caution when attempting to quit smoking, whether with or without medication. Smoking cessation has been linked to worsening underlying psychiatric conditions, such as depression.
Data from Champix smoking cessation studies included patients with a history of psychiatric disorders. In these clinical trials, neuropsychiatric adverse events were more commonly reported among patients with a psychiatric history, regardless of the treatment used.
Given this information, special care should be taken when treating patients with a history of psychiatric illness, and they should be advised accordingly during their smoking cessation journey.
Seizures
During clinical trials and post-marketing observations, some patients, both with and without a history of seizures, who were treated with this medicine, reported experiencing seizures. Therefore, it is essential to exercise caution when using Champix in patients with a history of seizures or other conditions that may lower the seizure threshold.
Treatment Discontinuation
Upon discontinuing treatment, up to 3% of patients may experience increased irritability, urge to smoke, depression, and/or insomnia. Therefore, prescribers must inform patients about this possibility and discuss the potential need for dose tapering when ending the treatment.
Cardiovascular Events
Patients taking this drug should be instructed to notify their doctor of new or worsening cardiovascular symptoms and to seek immediate medical attention if they experience signs and symptoms of myocardial infarction or stroke.
Hypersensitivity Reactions
Post-marketing reports of hypersensitivity reactions, including angioedema, have been in patients treated with varenicline. Clinical signs included swelling of the face, mouth (tongue, lips, and gums), neck (throat and larynx), and extremities. There were rare reports of life-threatening angioedema requiring urgent medical attention due to respiratory compromise. Patients experiencing these symptoms should discontinue treatment with varenicline and contact a healthcare provider immediately.
Cutaneous Reactions
There have also been post-marketing reports of rare but severe cutaneous reactions, including Stevens-Johnson Syndrome and Erythema Multiforme in patients using varenicline. As these skin reactions can be life-threatening, patients should discontinue treatment at the first sign of a rash or skin reaction and contact a healthcare provider immediately.
Contraindications
Individuals with hypersensitivity to the active substance or any of the ingredients should not use this medication.
Interactions
Here is the information about Champix interactions with the most popular medicines.
Metformin
Varenicline had no impact on the pharmacokinetics of metformin, and metformin did not affect varenicline's pharmacokinetics.
Cimetidine
When cimetidine was co-administered with varenicline, it led to a 29% increase in varenicline's systemic exposure due to reduced varenicline renal clearance. No dose adjustment is recommended when cimetidine is given with varenicline in individuals with normal renal function or those with mild to moderate renal impairment. However, the concurrent use of cimetidine and varenicline should be avoided in patients with severe renal impairment.
Digoxin
Varenicline had no impact on the steady-state pharmacokinetics of digoxin.
Warfarin
Varenicline did not affect the pharmacokinetics of warfarin, and prothrombin time (INR) remained unaffected by varenicline. It is worth noting that smoking cessation itself can cause changes to warfarin pharmacokinetics.
Alcohol
There is limited clinical data on the interaction between alcohol and varenicline. However, some post-marketing reports suggest increased intoxicating effects of alcohol in patients treated with varenicline. The causal relationship between these events and varenicline use has not been established.
Use with Other Therapies for Smoking Cessation
Bupropion
Varenicline did not influence the steady-state pharmacokinetics of bupropion.
Nicotine replacement therapy (NRT)
When varenicline and transdermal NRT were administered together to smokers for 12 days, there was a statistically significant decrease in average systolic blood pressure (mean 2.6 mmHg) on the final day of the study. The combination showed a higher incidence of nausea, headache, vomiting, dizziness, dyspepsia, and fatigue compared to NRT alone. The safety and efficacy of this drug in combination with other smoking cessation therapies have not been studied.
Fertility, Pregnancy, and Lactation
Pregnancy: While a moderate amount of data on pregnant women did not show any malformations or fetal/neonatal toxicity related to varenicline, animal studies have indicated reproductive toxicity. As a precautionary measure, avoiding using varenicline during pregnancy is advisable.
Breastfeeding: The presence of varenicline in human breast milk is unknown, but animal studies suggest it is excreted in breast milk. When considering whether to continue or discontinue breastfeeding or Champix therapy, the potential benefits of breastfeeding for the child and the benefits of Champix treatment for the woman should be carefully weighed.
Fertility: There is no available clinical data on the effects of varenicline on fertility in humans. However, non-clinical studies in rats showed no adverse effects on standard male and female fertility.
Effects on the Ability to Drive and Use Machines
Champix may have a slight to moderate impact on the ability to drive and operate machinery. It can cause dizziness, sleepiness, and temporary loss of consciousness, potentially affecting the capability to engage in such activities. Patients are cautioned against driving, operating complex machinery, or participating in other hazardous tasks until they determine how this medication affects their ability to perform these activities. Safety should be prioritized until the individual's response to Champix is fully understood.
Overdose
During pre-marketing clinical trials, there were no reported overdose cases with varenicline. In an overdose, standard supportive measures should be implemented as needed. Varenicline has been observed to be dialyzed in patients with end-stage renal disease. However, there is no specific experience with dialysis following an overdose of varenicline.
Storage
Store Champix tablets at room temperature, typically between 20°C to 25°C (68°F to 77°F). Keep the medication away from moisture; do not store it in the bathroom or any other area with high humidity. Store the tablets in their original packaging or a tightly closed container that protects them from light exposure. Ensure that Champix is stored securely, out of reach of children and pets. Avoid transferring the tablets to another container, especially if it is not child-resistant.
